URGENT NOTIFICATION
| Potentially affected Product name | Product no. | Lots | UPC |
|---|---|---|---|
| Accu-Chek Aviva test strips (50 tests) | 06453970164 | 498711, 498893, 498951, 498969 | 064562 31234 6 |
| Accu-Chek Aviva test strips (100 tests) | 06453988164 | 498583, 498586, 498688, 498711, 498717, 498760, 498769, 498832, 498845, 498846, 498871, 498894, 498945, 498984, 499095, 499111, 500023, 500039 | 064562 31235 3 |
According to Health Canada Medical Device Regulation, we are required to provide the following information to all potential users. Additionally, in order to comply with this regulation, an answer to this urgent notification is required by October 15th, 2021. Please refer to the section Action required. According to Health Canada Medical Device Regulation, an answer to this Urgent Notification is required and mandatory.
I attest that I have read the document and understand the issue at hand.
At Roche Diabetes Care we hold our products to the highest standards of quality and are committed to communicating any issues impacting our products. This is why we would like to inform you that as part of our ongoing quality monitoring and market surveillance processes, we have identified that, in rare cases Accu-Chek Aviva test strips vials have opened while still in a sealed carton.
If you have a vial that has opened while still in a sealed carton, you may observe the inability to perform a valid blood glucose measurement on the meter, because an open vial would expose the test strips to humidity which damages the strips and could result in inaccurate results (such as positively biased, or falsely too high, results).
Description of the situation
Roche has received complaints from one hospital in the United States alleging unexpected results (such as positively biased, or falsely too high, results) when using test strip vials which have opened while still in a sealed carton. The Roche investigation showed that, in very rare circumstances, it is possible that a vial can open in a sealed carton while in transit.
This could happen to Accu-Chek Aviva test strips, when they are shipped at elevated temperature (≥45°C or 113°F) AND when the carton is dropped or handled roughly during the transit and distribution process. It is only when these two conditions occur in combination that the issue has been observed. Due to the influence of high temperature and humidity, this issue might affect the accuracy of the blood glucose measurement.
Action taken by Roche Diabetes Care
Roche Diabetes Care is updating the product labelling to clarify instructions for handling vials that have opened within sealed cartons.
Instructions for users
- Always check vials of Accu-Chek Aviva test strips before use.
- Please, do not use the test strips if:
- vial is open or damaged before using the test strips for the first time,
- the cap is not fully closed,
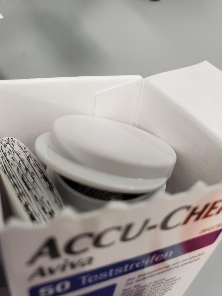
- you see any damage to the cap or vial, or
- anything prevents the cap from closing properly.
- Please do not perform control testing as instructed in the meter manual.
NOTE: this verification must be done for all vials/all lots from now on.
Action required
If you have any vial that was opened within the sealed carton, do not use the test strips, call Accu-Chek Customer Care line at 1-800-363-7949 or email [email protected] and dispose of the product.
In order to comply with Health Canada Medical Device Regulations you must confirm that you have read and understood this Urgent Notification by clicking on "I attest that I have read the document and understand the issue at hand."
Your confirmation must be sent no later than October 15th, 2021.
I attest that I have read the document and understand the issue at hand.
Questions
Should you need more information, please do not hesitate to contact Accu-Chek Customer Care line at 1-800-363-7949 or also by e-mail at [email protected].
